 General
General
Our latest collaboration with the SUNCAT Center (Stanford) has…
Our paper, Copper Silver Thin Films with Metastable Miscibility for Oxygen Reduction Electrocatalysis in Alkaline Electrolytes, has just been published in the journal ACS Applied Energy Materials. It has been a pleasure to work with our collaborators at Stanford once again! You can find the abstract of the paper below.
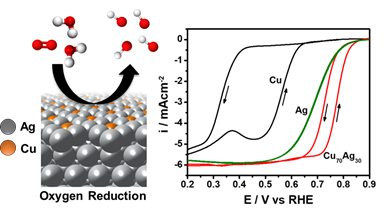
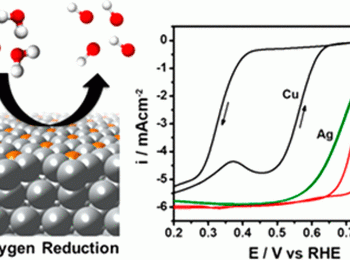
Increasing the activity of Ag-based catalysts for the oxygen reduction reaction (ORR) is important for improving the performance and economic outlook of alkaline-based fuel cell and metal-air battery technologies. In this work, we prepare CuAg thin films with controllable compositions using electron beam physical vapor deposition. X-ray diffraction analysis indicates that this fabrication route yields metastable miscibility between these two thermodynamically immiscible metals, with the thin films consisting of a Ag-rich and a Cu-rich phase. Electrochemical testing in 0.1 M potassium hydroxide showed significant ORR activity improvements for the CuAg films. On a geometric basis, the most active thin film (Cu70Ag30) demonstrated a 4-fold activity improvement vs pure Ag at 0.8 V vs the reversible hydrogen electrode. Furthermore, enhanced ORR kinetics for Cu-rich (>50 at. % Cu) thin films was demonstrated by a decrease in Tafel slope from 90 mV/dec, a commonly observed value for Ag catalysts, to 45 mV/dec. Surface enrichment of the Ag-rich phase after ORR testing was indicated by X-ray photoelectron spectroscopy and grazing incidence synchrotron X-ray diffraction measurements. By correlating density functional theory with experimental measurements, we postulate that the activity enhancement of the Cu-rich CuAg thin films arises due to the non-equilibrium miscibility of Cu atoms in the Ag-rich phase, which favorably tunes the surface electronic structure and binding energies of reaction species.